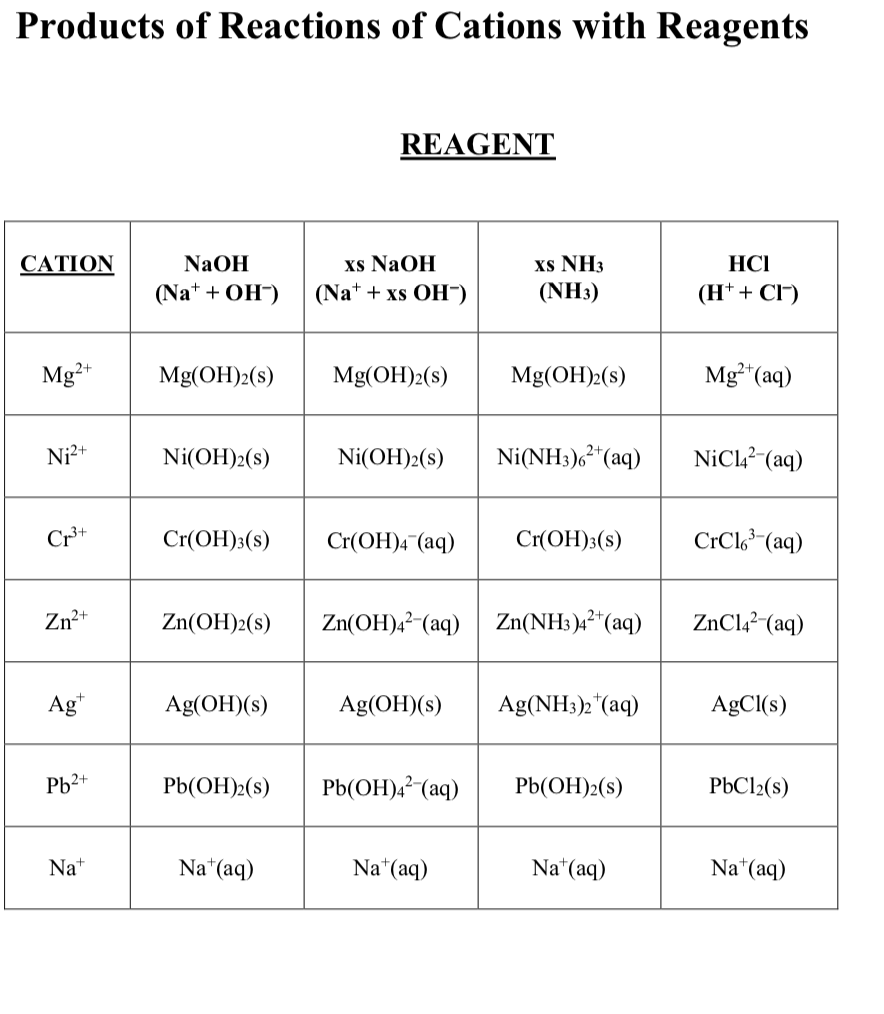
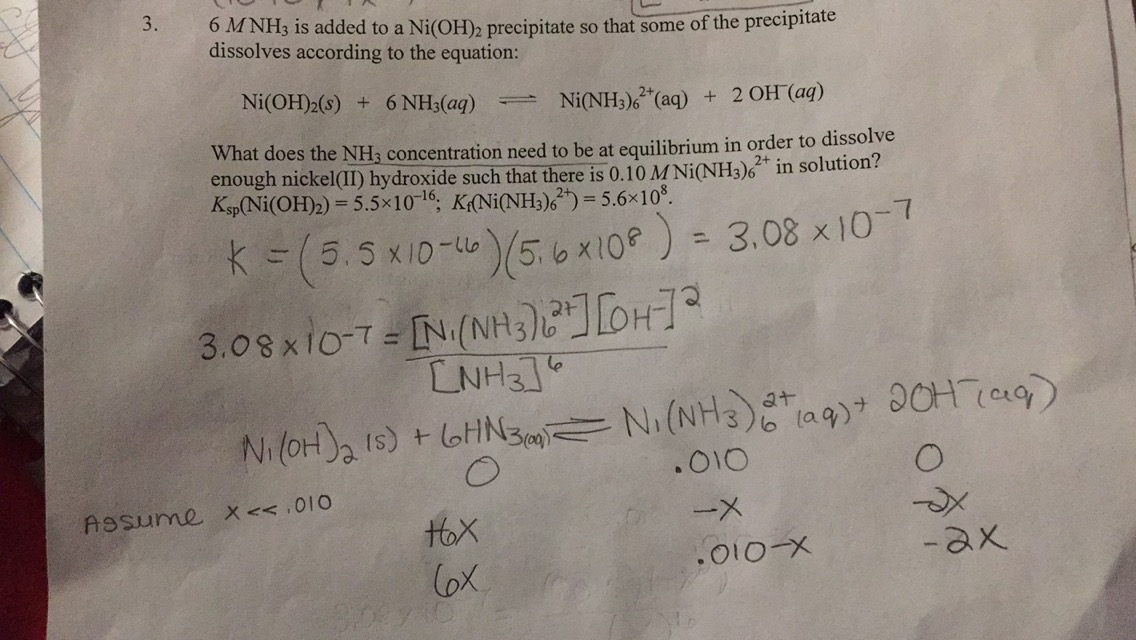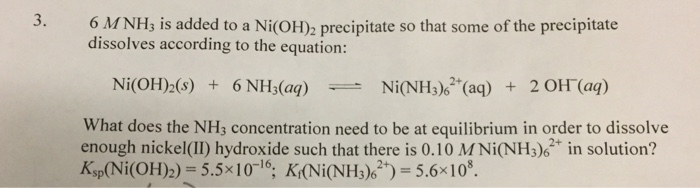Amongst the following, the total number of compounds soluble in concentrated NH3 solution is: Ag2CrO4,Cu(OH)2, PbSO3, Al(OH)3, Ni(OH)2, Zn3(PO4)2,BaSO4

Inorganics | Free Full-Text | Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate

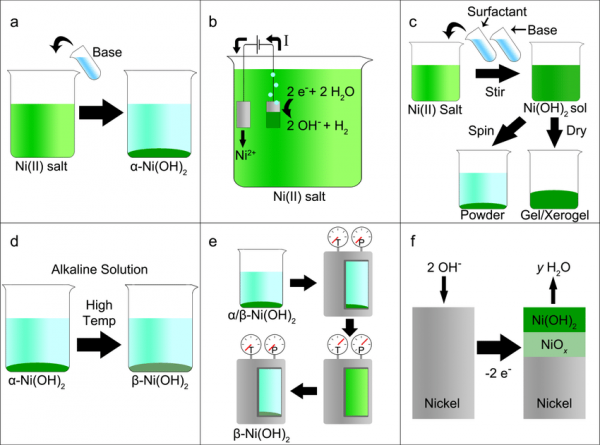
Triple Functions of Ni(OH)2 on the Surface of WN Nanowires Remarkably Promoting Electrocatalytic Activity in Full Water Splitting | ACS Catalysis

Porous Fe-Doped β-Ni(OH)2 Nanopyramid Array Electrodes for Water Splitting | ACS Applied Materials & Interfaces

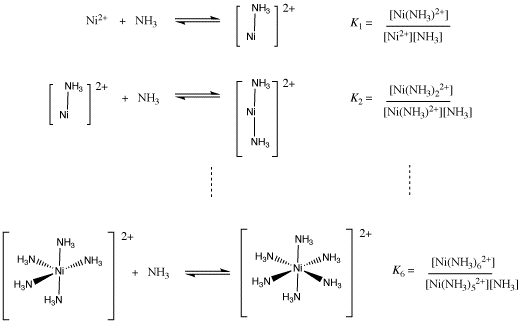
Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes

Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes

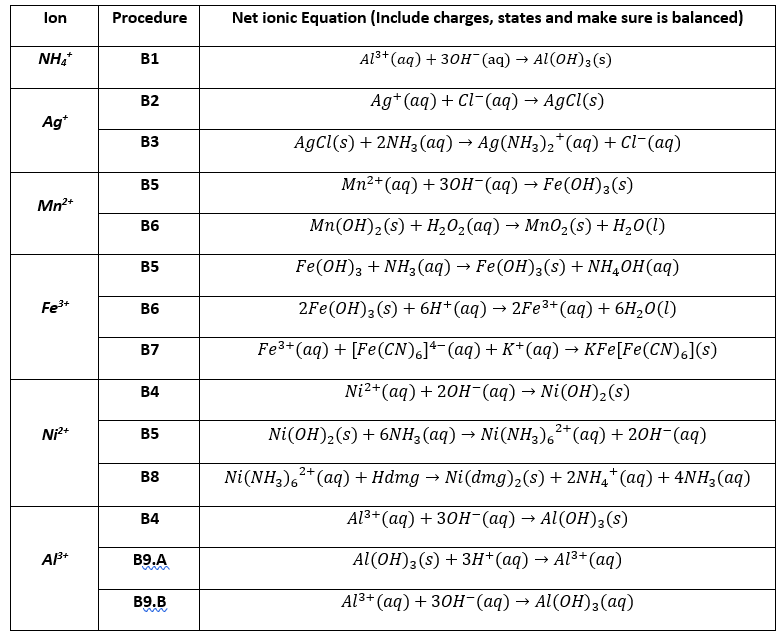
SOLVED: Questions Write net ionic equations for the reaction of nickel(ll) chloride with 6M NaOH t0 give Ni(OH)z: the reaction of iron(III) nitrate with 6M NaOH to give iron(IlI) hydroxide The reaction

SEM images of various electrocatalysts synthesized in water and ammonia... | Download Scientific Diagram
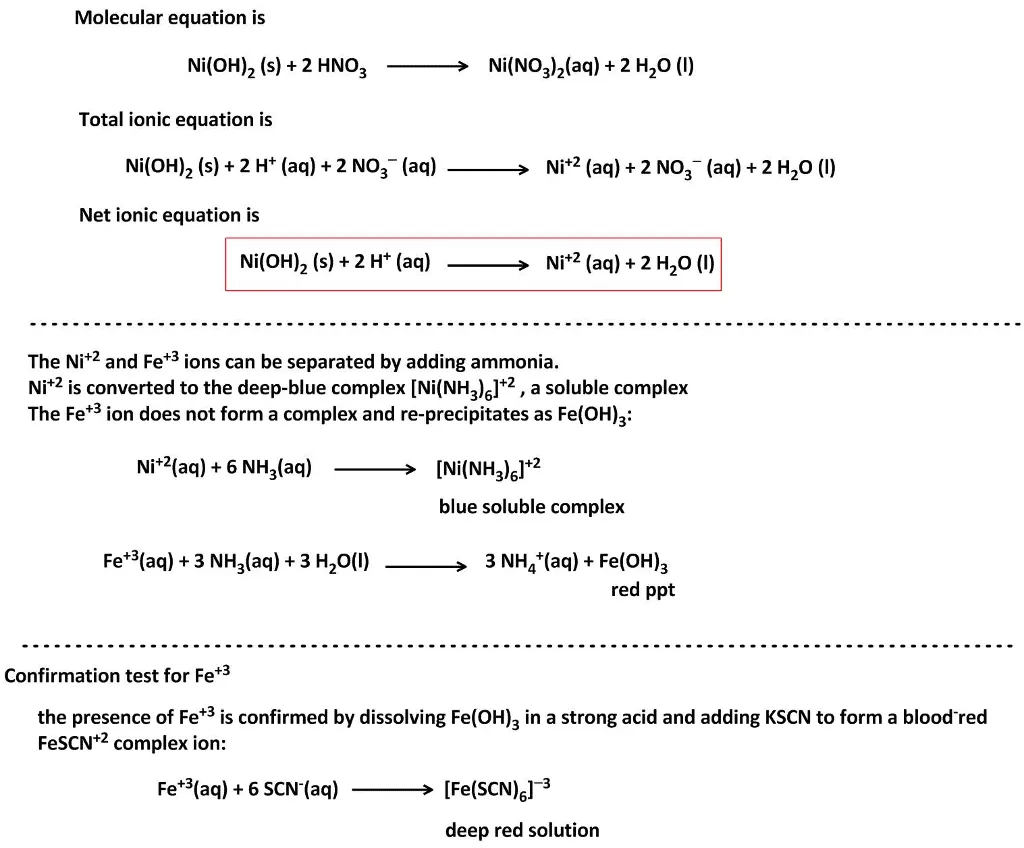
OneClass: write a balanced net ionic equation for A. dissolving of Ni (OH)2 in nitric acid. B. Ni 2+ ...

Electrocatalytic ammonia oxidation over a nickel foam electrode: Role of Ni( OH)2(s)-NiOOH(s) nanocatalysts - ScienceDirect

Electrocatalytic ammonia oxidation over a nickel foam electrode: Role of Ni( OH)2(s)-NiOOH(s) nanocatalysts - ScienceDirect

Stability-Enhanced α-Ni(OH)2 Pillared by Metaborate Anions for Pseudocapacitors | ACS Applied Materials & Interfaces