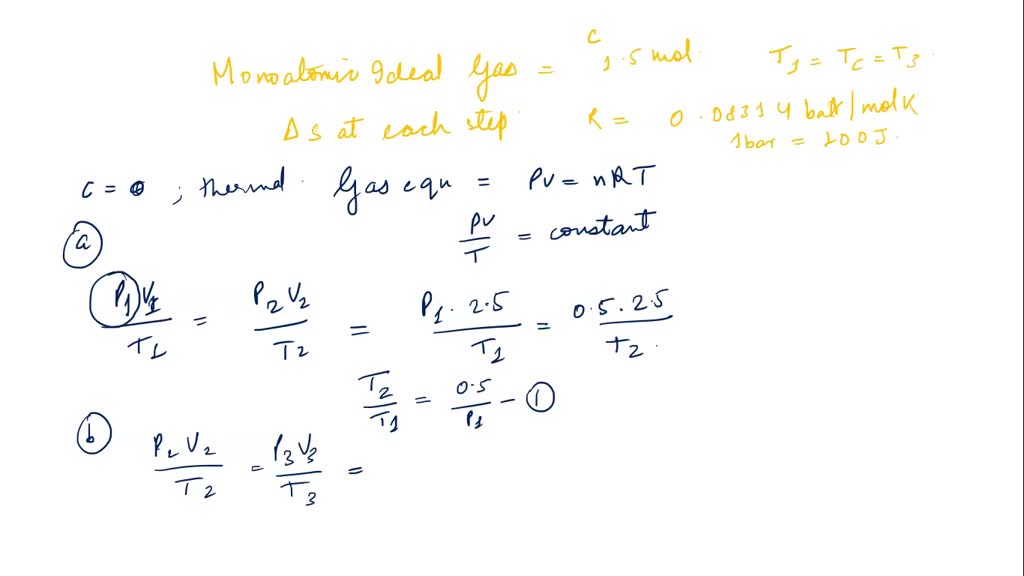
SOLVED: The molecular dlameter of CO Is 3.19x 10 " cm: At 600 K and 3 pressure of 200 mm Hg what will the number of molecules colliding per cubic centimeter per

SOLVED: The molecular dlameter of CO Is 3.19x 10 " cm: At 600 K and 3 pressure of 200 mm Hg what will the number of molecules colliding per cubic centimeter per

SOLVED: Consider the following samples of gas: sample composition pressure temperature 2.1 mol Ar 1.7 atm 282 "C sample composition pressure temperature 1.9 mol Ne 2.3 atm 1.1 mol Ar 1.7 atm
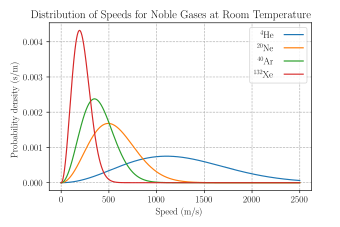
27.3: The Distribution of Molecular Speeds is Given by the Maxwell-Boltzmann Distribution - Chemistry LibreTexts

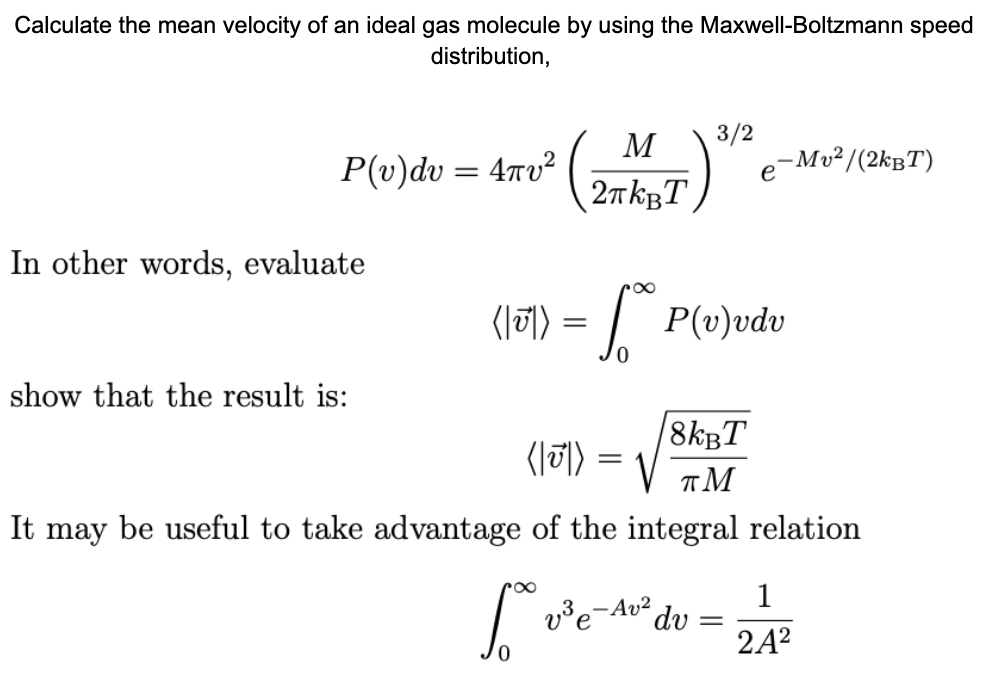
The Maxwell-Boltzmann distribution of molecular speeds in a sample of an ideal gas can be expressed as f=(4)/(sqrt(pi))((m)/(2kT))^(3//2)v^(2)e^(-(mv^(2))/(2kT)).dv Where f represent the fraction of total molecules that have speeds between v and
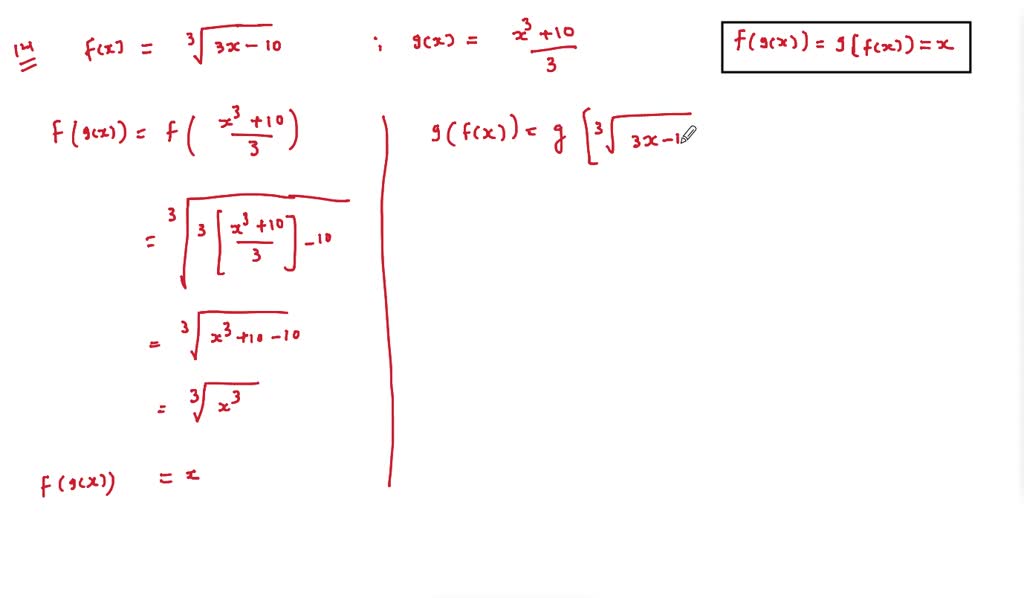
SOLVED:In Exercises 9-14, (a) show that f and g are inverse functions algebraically and (b) use a graphing utility to create a table of values for each function to numerically show that

Coefficients of Cauchy dispersion equation of PIMNT single crystals... | Download Scientific Diagram
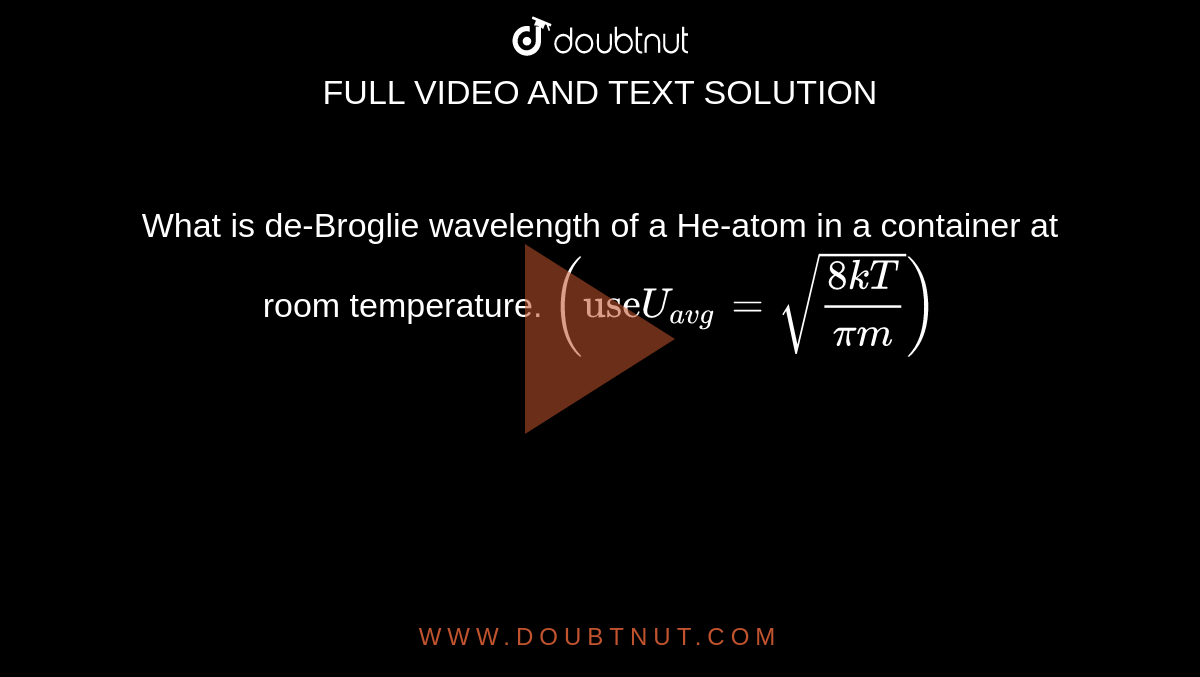
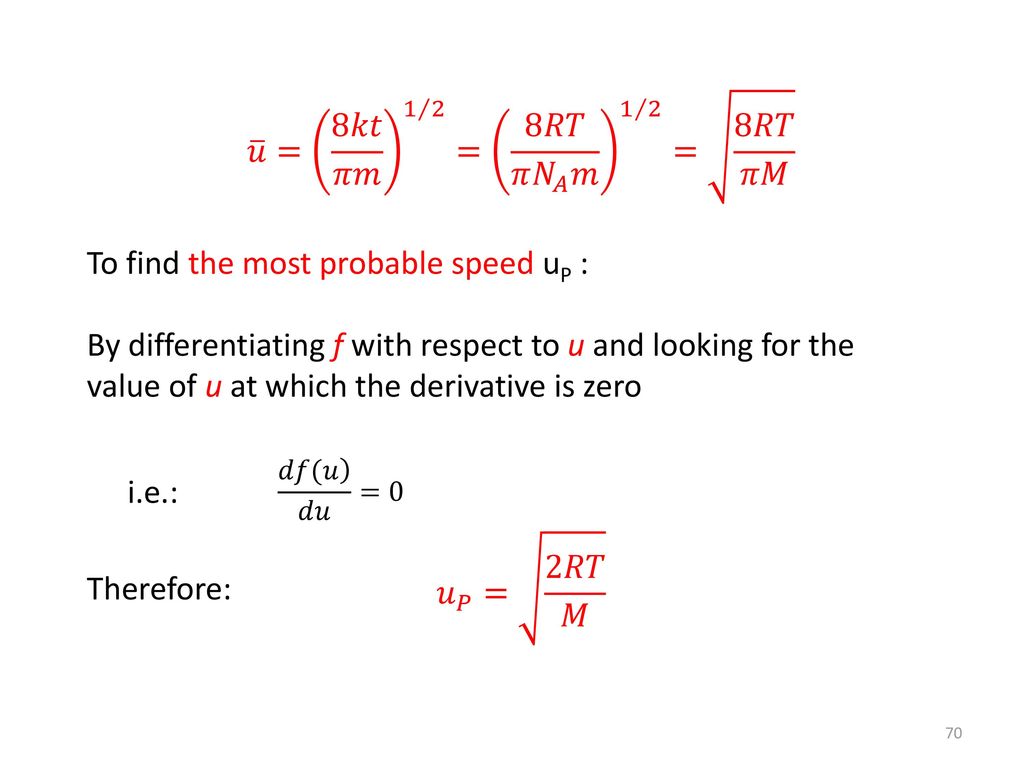
What is de-Broglie wavelength of a He-atom in a container at room temperature. ("use"U(avg)=sqrt((8kT)/(pim)))
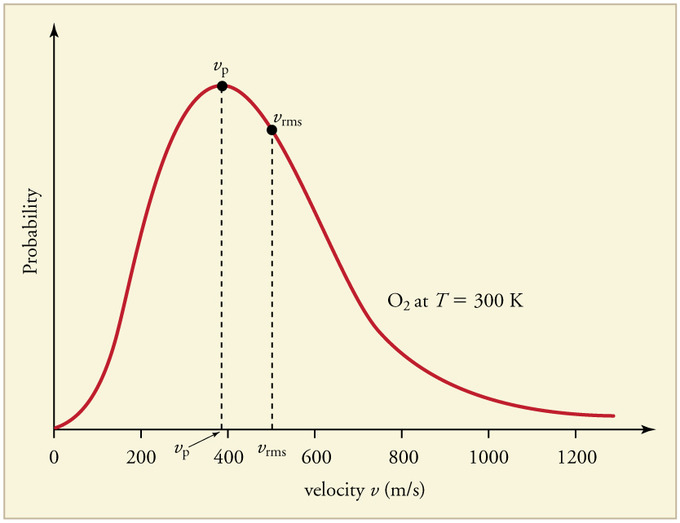
Show that the average speed of gas molecule <ν>= √(8kT/πm). - Sarthaks eConnect | Largest Online Education Community

SOLVED: What, according to the Maxwell-Boltzmann distribution, is the proportion of gas molecules having (a) more than, (b) less than the root mean square speed? (c) what are the proportions having speed